Abstract
Background Conditioning regimen plays a crucial role in determining the outcomes of patients with hematological malignancy treated with allogeneic hematopoietic stem cell transplantation (HSCT). In a multicenter, international, randomized, phase 3 clinical Trial (FORUM study), we documented (Peters C, et al. J Clin Oncol 2021) that children with acute lymphoblastic leukemia (ALL) given total body irradiation (TBI) in combination with etoposide had a superior probability of 2-year overall (OS) and event-free survival (EFS) in comparison to patients given either one of two myeloablative chemo-conditioning regimens. In this report, we assessed whether the benefit deriving from the use of TBI was sustained over time.
Patients and methods Patients <18 years at diagnosis, 4-21 years at HSCT, in complete morphological remission (CR) pre-HSCT, and with an HLA-compatible related or unrelated donor (UD) were randomly assigned 1:1 to a myeloablative conditioning regimen with fractionated TBI and etoposide or fludarabine, thiotepa, and either busulfan (BU) or treosulfan (TREO). Children transplanted from a matched sibling donor (MSD) received cyclosporine A only as graft-versus-host disease (GvHD) prophylaxis, whereas recipients of UD HSCT also received short-term methotrexate and anti-thymocyte globulin (ATG). Further details on transplant procedure have been described elsewhere (Peters C, et al. J Clin Oncol 2021).
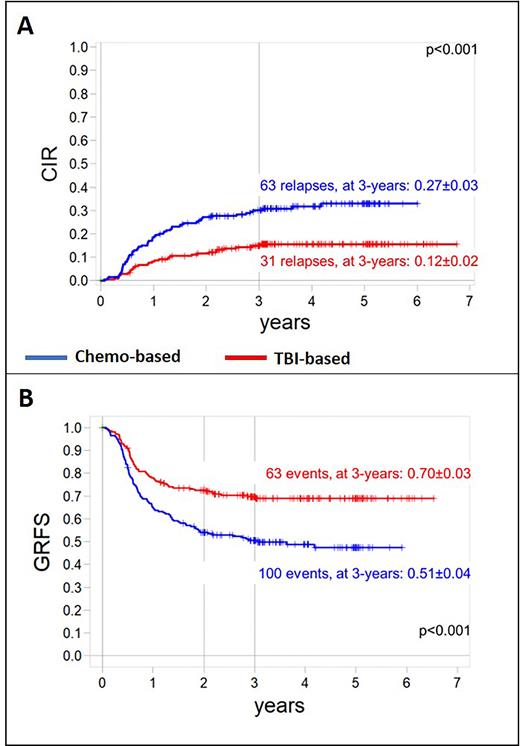
Results Between 04/2013 and 12/2018, 413 patients were randomly assigned to receive either TBI (212) or a chemotherapy-based conditioning (201). Compliance with random assignment was 92%. Sixty-five percent of patients were male, 72% had B-cell precursor ALL, 73% were transplanted from a UD, and in 82% of patients bone marrow was the stem cell source employed. Fifty-four percent of patients were in CR1 at time of allograft, while 40% and 4% were transplanted in CR2 and CR3, respectively. Patient's outcomes were updated on February 1st, 2022. With a median follow-up of 3.7 years (range, 0.3-7.9), the 3-year probability of OS of patients allocated to TBI/etoposide or to the chemotherapy-based conditioning regimens was 90±2% and 71±3% (p<0.001), respectively, while that of 3-year EFS was 81±3% and 59±4% (p<0.001). OS and EFS remain comparable in patients receiving BU or TREO in combination with fludarabine and thiotepa. In detail, OS and EFS of the 102 patients given BU, fludarabine and thiotepa were 73±5% and 69±5% respectively, while those of the 99 patients treated with TREO, fludarabine and thiotepa were 62±5% and 61±5%. Multivariate analysis confirmed that the use of chemotherapy-based regimens correlated with worse OS [Hazard Ratio, HR, 2.63, 95% confidence interval, CI, (1.61- 4.31), p<0.001] and EFS [HR 2.30, 95%CI (1.58- 3.35) p<0.001]. Transplantation in >CR1 predicted a worse EFS in multivariate analysis, as well [HR 1.67, 95% CI (1.14- 2.45) p=0.009]. Patients given TBI benefited from a lower cumulative incidence of relapse (Figure 1A), as well as from a lower 3-year cumulative incidence of non-relapse mortality (2+1% vs. 10+2% in the chemo arm, p=0.007). The 3-year cumulative incidence of chronic GvHD was comparable between the 2 arms (15+3% vs. 12+2% in the TBI and chemo arm, respectively, p=n.s.). We also analyzed the probability of GvHD/relapse-free survival (GRFS) considering both relapse and chronic GvHD as events; children prepared with TBI had a better 3-year GRFS probability (Figure 1B) as compared to chemo-prepared patients. Five patients (all transplanted from an UD) developed secondary malignancies: 4 of them had received TBI.
Conclusions With a significantly longer follow-up, we confirm that the use of TBI during the conditioning regimen is associated with better outcomes in children and adolescents with ALL above the age of 4 years transplanted in CR, mainly due to a lower risk of leukemia recurrence. The number of cases of secondary malignancies, particularly in patients offered radiotherapy, deserves particular attention and underlines the importance of continuous safety monitoring over time to comprehensively evaluate the long-term sequels observed in this population.
Disclosures
Locatelli:NEOVII: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANOFI: Membership on an entity's Board of Directors or advisory committees; MILTENYI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MEDAC: Speakers Bureau; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Membership on an entity's Board of Directors or advisory committees; JAZZ PHARMACEUTICALS: Speakers Bureau; SOBI: Speakers Bureau; TAKEDA: Speakers Bureau; GILEAD: Speakers Bureau; BLUEBIRD BIO: Speakers Bureau. Bader:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Research Funding; Neovii: Research Funding; Riemser: Research Funding, Speakers Bureau; Medac: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Jazz: Speakers Bureau; Miltenyi: Speakers Bureau. Dalle:Teva: Current equity holder in private company; Medac: Honoraria; Orchard: Honoraria; Vertex: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria. Büchner:Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ifversen:Novartis: Membership on an entity's Board of Directors or advisory committees. Peters:Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Medac: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Riemser: Consultancy, Speakers Bureau; Jazz: Other, Research Funding; Neovii: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.